Pappo Research Group
Organic Chemistry at Ben-Gurion University of the Negev
48. Ortho–Ortho Selective Oxidative Coupling of Phenols by Hydroxo Multicopper(II) Clusters
Ratnadeep Bera; Omer Shaashua; Anna Libman; Max T.G.M. Derks; Bowei Yuan; Aleksandr Y. Pereverzev; Raja Kapanaiah; Mor Ben Lulu; Lina Kertzman; Yulia Vainer; Yury V. Torubaev; Jana Roithová; Doron Pappo
J. Am. Chem. Soc., 2025, Article ASAP. DOI: 10.1021/jacs.5c10379

47. Halogen Bond-Driven Ligand Displacement: Co-Crystal Lattice Versus Coordination Bonds
Yury V. Torubaev,* Omer Shaashua, Savion Braunstein, Doron Pappo
Chem. - Eur. J. 2025, 31 (26), e202404784.

46. Twisting the Propotypic Molecules of Ph–C≡C–Ph, Ph–CH═CH–Ph, and Ph–N═N–Ph through Cocrystallization
Yury V. Torubaev,* Ivan V. Skabitskiy, Mariela J. Pavan, and Doron Pappo
Crystal Growth & Design 2025, 5742-5751.

45. Chiral Bis-8-Aryl-isoquinoline Bis-alkylamine Iron Catalysts for Asymmetric Oxidation Reactions
Tomer Mitz, Lei Liu and Doron Pappo*
Org. Lett. 2025, 27 (4), 1078-1083.

44. Aerobic Oxidative Coupling of 2‑Aminonaphthalenes byHomogenous Nonheme Iron Catalysts
Vlada Vershinin, Li-noy Feruz, Hagit Forkosh, Lina Kertzman, Anna Libman, Jordi Burés* and Doron Pappo*
ACS Catal. 2024, 14, 8261−8269.

43. Dynamic Thermodynamic Resolution of Racemic 1,1′-Binaphthyl-2,2′-diol (BINOL)
Omer Shaashua, Dennis Pollok, Alina Dyadyuk, Alexander I. Shames, Siegfried R. Waldvogel*, and Doron Pappo*
Org. Lett. 2024, 26, 10, 2129–2134.

42. Multicopper Clusters Enable Oxidative Phenol Macrocyclization (OxPM) of Peptides
Anna Libman, Mor Ben-Lulu, Eden Gaster, Ratnadeep Bera, ALexander I. Shames, Omer Shaashua, Vlada Vershinin, Yuri Torubaev and Doron Pappo
J. Am. Chem. Soc. 2023, 145 (38), 21002-21011.
https://pubs.acs.org/doi/full/10.1021/jacs.3c06978
Published first in ChemRxiv, preprint 2023

41. A Chiral Iron Disulfonate Catalyst for the Enantioselective Synthesis of 2-Amino-2′-hydroxy-1,1′-binaphthyls (NOBINs)
Alina Dyadyuk, Vlada Vershinin, Hadas Shalit, Hen Shalev, Nagnath Yadav More, and Doron Pappo
J. Am. Chem. Soc. 2022, 144 (8), 3676-3684.

40. Iron-Catalyzed Oxidative Cross-Coupling of Phenols and Tyrosine Derivatives with 3-Alkyloxindoles
Tomer Mintz, Nagnath Yadav More, Eden Gaster and Doron Pappo
J. Org. Chem. 2021, 86, 24, 18164–18178.

Vlada Vershinin, Hagit Forkosh, Mor Ben-Lulu, Anna Libman and Doron Pappo
J. Org. Chem. 2020, 86,1, 79-90.

38. Flat corannulene: when a transition state becomes a stable molecule
Ephrath Solel, Doron Pappo, Ofer Reany, Tom Mejuch, Renana Gershoni-Poranne, Mark Botoshansky, Amnon Stanger and Ehud Keinan Chem. Sci. 2020, 11, 13015-13025.

38. Flat corannulene: when a transition state becomes a stable molecule
Ephrath Solel, Doron Pappo, Ofer Reany, Tom Mejuch, Renana Gershoni-Poranne, Mark Botoshansky, Amnon Stanger and Ehud Keinan Chem. Sci. 2020, 11, 13015-13025.

37. Dual‐Acting Small‐Molecule Inhibitors Targeting Mycobacterial DNA Replication
Meenakshi Singh, Stefan Ilic, Benjamin Tam, Yesmin Ben-Ishay, Dror Sherf, Doron Pappo and Barak Akabayov
Chem. Eur. J. 2020, 26, 10849-10860
37. Dual‐Acting Small‐Molecule Inhibitors Targeting Mycobacterial DNA Replication
Meenakshi Singh, Stefan Ilic, Benjamin Tam, Yesmin Ben-Ishay, Dror Sherf, Doron Pappo and Barak Akabayov
Chem. Eur. J. 2020, 26, 10849-10860
36. M[TPP]Cl (M = Fe or Mn)-Catalyzed Oxidative Amination of Phenols by Primary and Secondary Anilines
Vlada Vershinin and Doron Pappo Org. Lett. 2020, 22, 5, 1941-1946
36. M[TPP]Cl (M = Fe or Mn)-Catalyzed Oxidative Amination of Phenols by Primary and Secondary Anilines
Vlada Vershinin and Doron Pappo Org. Lett. 2020, 22, 5, 1941-1946

35. Synthesis of Biaryl‐Bridged Cyclic Peptides via Catalytic Oxidative Cross‐Coupling Reactions
Mor Ben-Lulu, Eden Gaster, Anna Libman, and Doron Pappo Angew. Chem. Int. Ed. 2020, 59 (12), 4835-4839
35. Synthesis of Biaryl‐Bridged Cyclic Peptides via Catalytic Oxidative Cross‐Coupling Reactions
Mor Ben-Lulu, Eden Gaster, Anna Libman, and Doron Pappo Angew. Chem. Int. Ed. 2020, 59 (12), 4835-4839
34. Cobalt(II)[salen] Catalyzed Selective Aerobic Oxidative Cross‑Coupling between Electron-Rich Phenols and 2-Naphthols
Hagai Reiss, Hadas Shalit, Vlada Vershinin, Nagnath Yadav More, Hagit Forckosha, and Doron Pappo J. Org. Chem. 2019, 84, 12, 7950-7960.
34. Cobalt(II)[salen] Catalyzed Selective Aerobic Oxidative Cross‑Coupling between Electron-Rich Phenols and 2-Naphthols
Hagai Reiss, Hadas Shalit, Vlada Vershinin, Nagnath Yadav More, Hagit Forckosha, and Doron Pappo J. Org. Chem. 2019, 84, 12, 7950-7960.
33. Selective Oxidative Phenol Coupling by Iron Catalysis
Hadas Shalit,† Alina Dyadyuk,† Doron Pappo J. Org. Chem. 2019, 1677-1686.
†These authors contributed equally to this work.
33. Selective Oxidative Phenol Coupling by Iron Catalysis
Hadas Shalit,† Alina Dyadyuk,† Doron Pappo J. Org. Chem. 2019, 1677-1686.
†These authors contributed equally to this work.
32. Stereoselective Synthesis of Optically Pure 2-Amino-2'-Hydroxy-1,1'-Binaphthyls [NOBINs]
Hagit Forkosh,† Vlada Vershinin,† Hagai Reiss, Doron Pappo Org. Lett. 2018, 20, 8, 2459-2463
DOI: 10.1021/acs.orglett.8b00800
†These authors contributed equally to this work.
32. Stereoselective Synthesis of Optically Pure 2-Amino-2'-Hydroxy-1,1'-Binaphthyls [NOBINs]
Hagit Forkosh,† Vlada Vershinin,† Hagai Reiss, Doron Pappo Org. Lett. 2018, 20, 8, 2459-2463
DOI: 10.1021/acs.orglett.8b00800
†These authors contributed equally to this work.
31. Organic Synthesis: From Glorious Past to Brilliant Future
Ehud Keinan, Doron Pappo Israel. J. Chem. 2018, 58, 7 – 10.
31. Organic Synthesis: From Glorious Past to Brilliant Future
Ehud Keinan, Doron Pappo Israel. J. Chem. 2018, 58, 7 – 10.
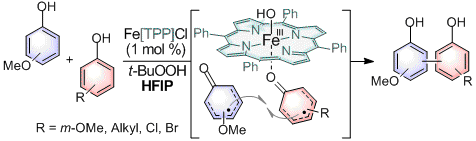
29. meso-Tetraphenylporphyrin Iron Chloride Catalyzed Selective Oxidative Cross-Coupling of Phenols
Hadas Shalit,† Anna Libman,† Doron Pappo J. Am. Chem. Soc. 2017, 139 (38), 13404–13413.
†These authors contributed equally to this work.
29. meso-Tetraphenylporphyrin Iron Chloride Catalyzed Selective Oxidative Cross-Coupling of Phenols
Hadas Shalit,† Anna Libman,† Doron Pappo J. Am. Chem. Soc. 2017, 139 (38), 13404–13413.
†These authors contributed equally to this work.
30. Cu(OTf)2 Catalyzed Pummerer Coupling of beta-Ketosulfoxide
Regev Parnes, Hagai Reiss and Doron Pappo J. Org. Chem. 2018, 83 (2), 723–732.
30. Cu(OTf)2 Catalyzed Pummerer Coupling of beta-Ketosulfoxide
Regev Parnes, Hagai Reiss and Doron Pappo J. Org. Chem. 2018, 83 (2), 723–732.
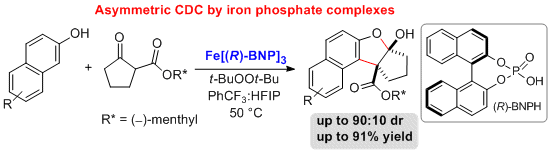
28. Iron Phosphate Catalyzed Asymmetric Cross-Dehydrogenative Coupling of 2-Napthols with beta-Ketoesters
Sachin Narute, Doron Pappo Org. Lett. 2017, 19 (11), 2917–2920.
28. Iron Phosphate Catalyzed Asymmetric Cross-Dehydrogenative Coupling of 2-Napthols with beta-Ketoesters
Sachin Narute, Doron Pappo Org. Lett. 2017, 19 (11), 2917–2920.
27. Selective Aerobic Oxidation of Methylarenes to Benzaldehydes Catalyzed by NHPI and Co(OAc)2 in HFIP
Eden Gaster, Sebastian Kozuch, Doron Pappo Angew. Chem. Int. Ed. 2017, 56, 5912-5915.
Selected as a Very Important Paper (VIP)
27. Selective Aerobic Oxidation of Methylarenes to Benzaldehydes Catalyzed by NHPI and Co(OAc)2 in HFIP
Eden Gaster, Sebastian Kozuch, Doron Pappo Angew. Chem. Int. Ed. 2017, 56, 5912-5915.
Selected as a Very Important Paper (VIP)
26. Iron-Catalyzed Selective Oxidative Arylation of Phenols and Biphenols
Vlada Vershinin, Alina Dyadyuk, Doron Pappo Tetrahedron 2017, 73(26), 3660-3668.
DOI: 10.1016/j.tet.2017.03.094
[Tetrahedron Young Investigator Prize Special Issue in honor of Professor Ang Li]
26. Iron-Catalyzed Selective Oxidative Arylation of Phenols and Biphenols
Vlada Vershinin, Alina Dyadyuk, Doron Pappo Tetrahedron 2017, 73(26), 3660-3668.
DOI: 10.1016/j.tet.2017.03.094
[Tetrahedron Young Investigator Prize Special Issue in honor of Professor Ang Li]
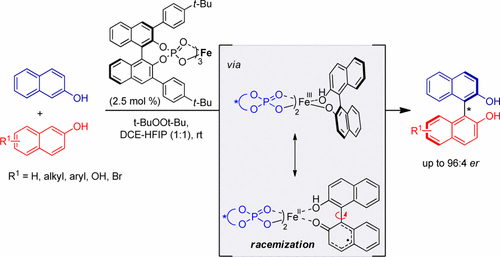
25. Enantioselective Oxidative Homo- and Cross-Coupling of 2-Naphthols Catalyzed by Chiral Iron Phosphate Complexes
Sachin Narute, Regev Parnes, F. Dean Toste, Doron Pappo J. Am. Chem. Soc. 2016, 138 (50), 16553–16560.
[Highlighted in SYNFACTS 2017, 273]
25. Enantioselective Oxidative Homo- and Cross-Coupling of 2-Naphthols Catalyzed by Chiral Iron Phosphate Complexes
Sachin Narute, Regev Parnes, F. Dean Toste, Doron Pappo J. Am. Chem. Soc. 2016, 138 (50), 16553–16560.
[Highlighted in SYNFACTS 2017, 273]
24. Direct Synthesis of Polyaryls by Consecutive Oxidative Cross-Coupling of Phenols with Arenes
Alina Dyadyuk, Kavitha Sudheendran, Yulia Vainer, Vlada Vershinin, Alexander I. Shames and Doron Pappo Org. Lett. 2016, 4324-4327.
24. Direct Synthesis of Polyaryls by Consecutive Oxidative Cross-Coupling of Phenols with Arenes
Alina Dyadyuk, Kavitha Sudheendran, Yulia Vainer, Vlada Vershinin, Alexander I. Shames and Doron Pappo Org. Lett. 2016, 4324-4327.
23. A Synthetic and Predictive Approach to Unsymmetrical Biphenols by Iron-Catalyzed Chelated Radical-Anion Oxidative Coupling
Anna Libman,† Hadas Shalit,† Yulia Vainer, Sachin Narute, Sebastian Kozuch and Doron Pappo J. Am. Chem. Soc. 2015, 11,453-11,460.
†These authors contributed equally to this work.
23. A Synthetic and Predictive Approach to Unsymmetrical Biphenols by Iron-Catalyzed Chelated Radical-Anion Oxidative Coupling
Anna Libman,† Hadas Shalit,† Yulia Vainer, Sachin Narute, Sebastian Kozuch and Doron Pappo J. Am. Chem. Soc. 2015, 11,453-11,460.
†These authors contributed equally to this work.
22. Reductive Alkylation of Arenes by a Thiol-Based Multicomponent Reaction
Regev Parnes and Doron Pappo Org. Lett. 2015, 2924-2927.
22. Reductive Alkylation of Arenes by a Thiol-Based Multicomponent Reaction
Regev Parnes and Doron Pappo Org. Lett. 2015, 2924-2927.
21. Iron Catalyzed Oxidative C-C and C-O Coupling of Halophenols to α-substituted β-ketoesters
Almog Regev, Hadas Shalit and Doron Pappo SYNTHESIS 2015, 47(12), 1716-1725.
[Special issue: Iron in Organic Synthesis]
21. Iron Catalyzed Oxidative C-C and C-O Coupling of Halophenols to α-substituted β-ketoesters
Almog Regev, Hadas Shalit and Doron Pappo SYNTHESIS 2015, 47(12), 1716-1725.
[Special issue: Iron in Organic Synthesis]
20. Significant Enhancement in Efficiency and Selectivity of Iron-Catalyzed Oxidative Cross-Coupling of Phenols by Fluoroalcohols
Eden Gaster,† Yulia Vainer,† Almog Regev, Sachin Narute, Kavitha Sudheendran, Aviya Werbeloff, Hadas Shalit and Doron Pappo Angew. Chem. Int. Ed. 2015, 54 (14), 4198-4202.
†These authors contributed equally to this work.
20. Significant Enhancement in Efficiency and Selectivity of Iron-Catalyzed Oxidative Cross-Coupling of Phenols by Fluoroalcohols
Eden Gaster,† Yulia Vainer,† Almog Regev, Sachin Narute, Kavitha Sudheendran, Aviya Werbeloff, Hadas Shalit and Doron Pappo Angew. Chem. Int. Ed. 2015, 54 (14), 4198-4202.
†These authors contributed equally to this work.
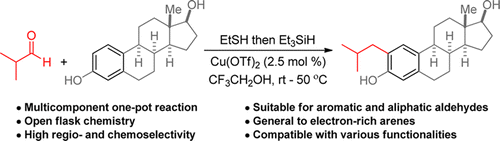
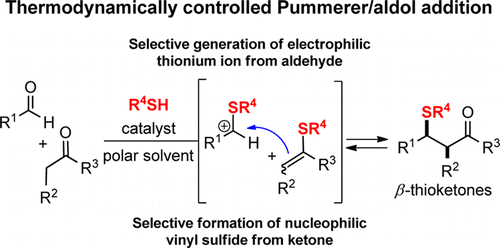
19. Thiol-Promoted Selective Addition of Ketones to Aldehydes
Regev Parnes,† Sachin Narute† and Doron Pappo Org. Lett. 2014, 16 (22), 5922–5925.
†These authors contributed equally to this work.
19. Thiol-Promoted Selective Addition of Ketones to Aldehydes
Regev Parnes,† Sachin Narute† and Doron Pappo Org. Lett. 2014, 16 (22), 5922–5925.
†These authors contributed equally to this work.
18. Multifarenes: New Modular Cavitands
Galit Parvari, Senthilmurugan Annamalai, Helena Chechik, Mark Botoshansky, Doron Pappo and Ehud Keinan Chem. Commun. 2014, 50, 2494-2497.
[Highlighted in SYNFACTS 2014, 375]
18. Multifarenes: New Modular Cavitands
Galit Parvari, Senthilmurugan Annamalai, Helena Chechik, Mark Botoshansky, Doron Pappo and Ehud Keinan Chem. Commun. 2014, 50, 2494-2497.
[Highlighted in SYNFACTS 2014, 375]
17. Copper-Mediated Cross Coupling Reactions
Edited by G. Evano and N. Blanchard, WILEY, 2013.
Doron Pappo, Chapter 17: Natural Products and C-O Bond Forming Reactions: Copper Showed the Way.
DOI: 10.1002/9781118690659.ch17
17. Copper-Mediated Cross Coupling Reactions
Edited by G. Evano and N. Blanchard, WILEY, 2013.
Doron Pappo, Chapter 17: Natural Products and C-O Bond Forming Reactions: Copper Showed the Way.
DOI: 10.1002/9781118690659.ch17
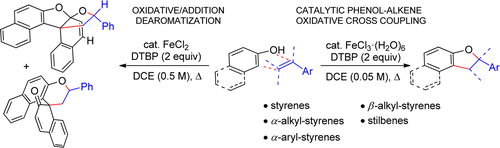
16. Iron-Catalyzed Oxidative Cross-Coupling of Phenols and Alkenes
Umesh A. Kshirsagar, Clil Regev, Regev Parnes and Doron Pappo Org. Lett., 2013, 15 (12), 3174-3177.
16. Iron-Catalyzed Oxidative Cross-Coupling of Phenols and Alkenes
Umesh A. Kshirsagar, Clil Regev, Regev Parnes and Doron Pappo Org. Lett., 2013, 15 (12), 3174-3177.
15. Aerobic Iron-Based Cross-Dehydrogenative Coupling Enables Efficient Diversity-Oriented Synthesis of Coumestrol-Based Selective Estrogen Receptor Modulators
Umesh A. Kshirsagar,† Parnes, Regev,† Hagit Goldshtein, Rivka Ofir, Raz Zarivach, Doron Pappo Chem. Eur. J., 2013, 19 (40), 13575-13583.
†These authors contributed equally to this work.
15. Aerobic Iron-Based Cross-Dehydrogenative Coupling Enables Efficient Diversity-Oriented Synthesis of Coumestrol-Based Selective Estrogen Receptor Modulators
Umesh A. Kshirsagar,† Parnes, Regev,† Hagit Goldshtein, Rivka Ofir, Raz Zarivach, Doron Pappo Chem. Eur. J., 2013, 19 (40), 13575-13583.
†These authors contributed equally to this work.
14. Ligand Controlled Iron-Catalyzed Coupling of a-Substituted β-Ketoesters with Phenols
Regev Parnes, Umesh A. Kshirsagar, A. Werbeloff, Clil Regev, Doron Pappo Org. Lett., 2012, 14(13), 3324-3327.
14. Ligand Controlled Iron-Catalyzed Coupling of a-Substituted β-Ketoesters with Phenols
Regev Parnes, Umesh A. Kshirsagar, A. Werbeloff, Clil Regev, Doron Pappo Org. Lett., 2012, 14(13), 3324-3327.
13. Deca-heterosubstituted corannulenes
Alla Pogoreltsev, Ephrath Solel, Doron Pappo, Ehud Keinan Chem. Commun., 2012, 48, 5425-5427.
13. Deca-heterosubstituted corannulenes
Alla Pogoreltsev, Ephrath Solel, Doron Pappo, Ehud Keinan Chem. Commun., 2012, 48, 5425-5427.
12. Corannulene Ethers via Ullmann Condensation
Renana Gershoni-Poranne, Doron Pappo, Ephrath Solel, Ehud Keinan Org. Lett., 2009, 11 (22), 5146-5149.
12. Corannulene Ethers via Ullmann Condensation
Renana Gershoni-Poranne, Doron Pappo, Ephrath Solel, Ehud Keinan Org. Lett., 2009, 11 (22), 5146-5149.
11. Diverse Functionalization of Corannulene: Easy Access to Pentagonal Superstructures
Doron Pappo, Tom Mejuch, Ofer Reany, Ephrath Solel, Ehud Keinan Org. Lett., 2009, 11 (5), 1063-1066.
11. Diverse Functionalization of Corannulene: Easy Access to Pentagonal Superstructures
Doron Pappo, Tom Mejuch, Ofer Reany, Ephrath Solel, Ehud Keinan Org. Lett., 2009, 11 (5), 1063-1066.
10. Cyclic Endiamino Peptides; A New Synthesis of Imidazopyrazines
Yael Kogon, Lee Goren, Doron Pappo, Amira Rudi, Yoel Kashman Eur. J. Org. Chem. 2009, 12, 1852-1854.
9. Acyclic and Cyclic Thioenamine peptides; Solution and Solid phase Synthesis
Lee Goren, Doron Pappo, Israel Goldberg, Yoel Kashman, Tetrahedron Letters, 2009, 50, 1048-1050.
8. A Chiral Pool Based Synthesis of Platensimycin
K.C. Nicolaou, Doron Pappo, K. Y. Tsang, Romelo Gibe, David Y.-K. Chen Angew. Chem. Int. Ed., 2008, 47(5), 944-946.
[Highlighted in SYNFACTS]
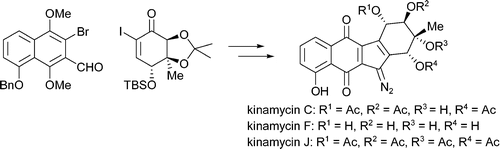
7. Total Synthesis of Kinamycins C, F and J
K.C. Nicolaou, Hongming Li, Andrean L. Nold, Doron Pappo, Achim Lenzen, J. Am. Chem. Soc., 2007, 129(34), 10356-10357.
7. Total Synthesis of Kinamycins C, F and J
K.C. Nicolaou, Hongming Li, Andrean L. Nold, Doron Pappo, Achim Lenzen, J. Am. Chem. Soc., 2007, 129(34), 10356-10357.
6. Recent Heterocyclic Compounds from Marine Invertebrates; Structure and Synthesis
Yoel Kashman, Amira Rudi, Doron Pappo Pure Appl. Chem. 2007, 79(4), 491-505.
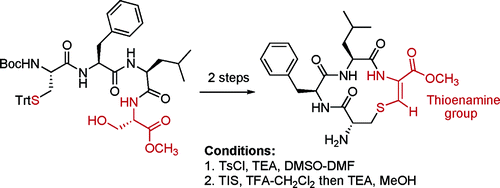
5. β-Turn Mimetic: Synthesis of Cyclic Thioenamino Peptides
Doron Pappo and Yoel Kashman Org. Lett., 2006, 8 (6), 1177-1179.
5. β-Turn Mimetic: Synthesis of Cyclic Thioenamino Peptides
Doron Pappo and Yoel Kashman Org. Lett., 2006, 8 (6), 1177-1179.
4. Synthesis of Cyclic Endiamino Peptides
Doron Pappo, Maida Vartanian, Stephan Lang, Yoel Kashman J. Am. Chem. Soc., 2005, 127 (21), 7682-7683.
4. Synthesis of Cyclic Endiamino Peptides
Doron Pappo, Maida Vartanian, Stephan Lang, Yoel Kashman J. Am. Chem. Soc., 2005, 127 (21), 7682-7683.
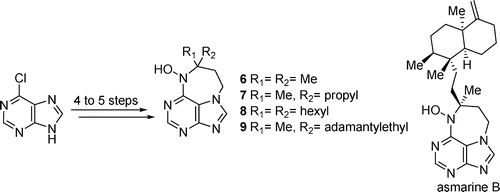
3. Synthesis of 9-Substituted Tetrahydrodiazepinopurines: Studies Toward the Total Synthesis of Asmarines
Doron Pappo, Shiri Shimony, Yoel Kashman J. Org. Chem., 2005, 70, 199-206.
5. β-Turn Mimetic: Synthesis of Cyclic Thioenamino Peptides
Doron Pappo, Yoel Kashman Org. Lett., 2006, 8(6), 1177-1179.
3. Synthesis of 9-Substituted Tetrahydrodiazepinopurines: Studies Toward the Total Synthesis of Asmarines
Doron Pappo, Shiri Shimony, Yoel Kashman J. Org. Chem., 2005, 70, 199-206.
2. Synthesis of 9-Substituted Tetrahydrodiazepinopurines- Asmarine A Analogues
Doron Pappo, Yoel Kashman, Tetrahedron, 2003, 59, 6493-6501.
DOI: 10.1016/S0040-4020(03)01058-5
2. Synthesis of 9-Substituted Tetrahydrodiazepinopurines- Asmarine A Analogues
Doron Pappo, Yoel Kashman, Tetrahedron, 2003, 59, 6493-6501.
DOI: 10.1016/S0040-4020(03)01058-5
1. A Synthetic Approach Toward the Synthesis of Asmarine Analogues
Doron Pappo, Amira Rudi, Yoel Kashman Tetrahedron Letters, 2001, 42, 5941-5943.
DOI: 10.1016/S0040-4039(01)01111-x